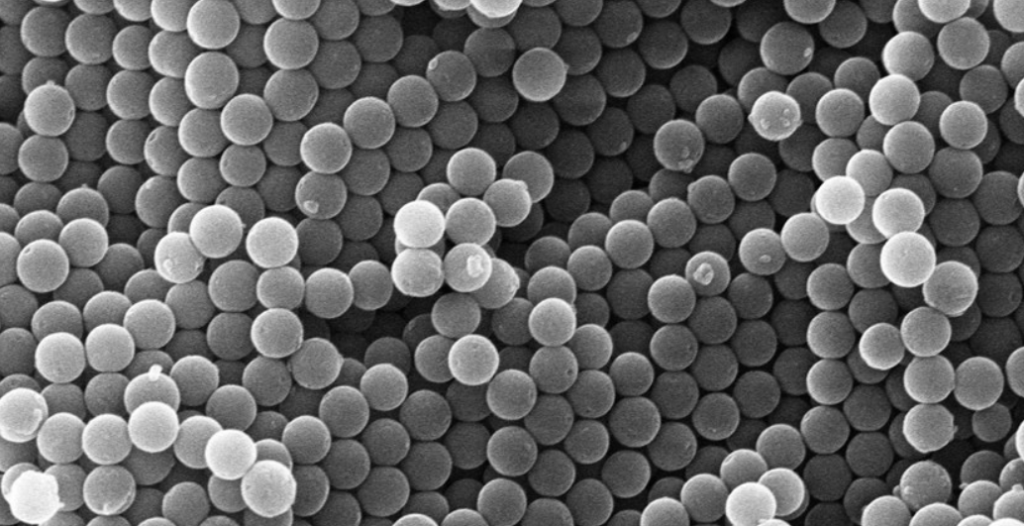
Silica microspheres are an important material, which is a hydrophilic milky white suspension. The silicon hydroxyl groups on the surface can covalently bond with other groups. It has stable physical and chemical properties, can withstand high temperatures up to 1000℃, and remains stable in organic solvents, but can dissolve in strong alkaline or HF solutions
Silica microspheres are non porous, spherical, and have very uniform sizes. The density of silica microspheres is 1.96 g/cm3, and they have been used to adsorb DNA and RNA from cell lysates. They can also provide functional groups such as epoxy, carboxylation, avidin, streptavidin, and protein A.
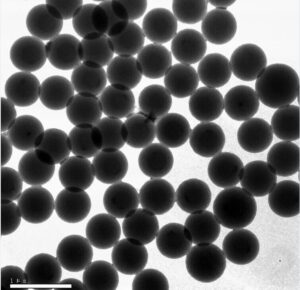
Silica microspheres have various excellent properties, such as controllable specific surface area, high mechanical strength, and stable thermal and physicochemical properties. Therefore, they have been widely used in catalyst carriers, adsorbent materials, chromatographic separation media, and other fields. The preparation and application research of silica microspheres is a hot topic in the field of materials research.
At the same time, the control based on the surface structure of silica can effectively enhance the specific surface area of microspheres and have many micro area and specific structural properties, effectively expanding the applications of silica microspheres in optics, drug delivery, self-assembly and other fields. In order to meet the growing demand for diversified applications, the preparation strategy of silica microspheres has gradually evolved from a single spherical structure to a multi-level structure with complex morphology.
At present, in the field of silica, two types of nanostructures are highly valued, one is hollow mesoporous silica microspheres; the other is monodisperse spherical SiO2. The former has a high specific surface area and pore volume, and is a good catalyst and drug carrier; the latter has a large specific surface area, good dispersibility, and good optical and mechanical properties, and has important applications in ceramics, coatings, optoelectronics and other fields.
At present, the preparation methods of silica microspheres can be mainly divided into two categories: dry method and wet method. The wet method includes sol-gel method, template method, precipitation method, supergravity reaction method, microemulsion method and hydrothermal synthesis method, while the dry method includes gas phase method and arc method.
Preparation method of silica microspheres
- Sol-gel method
The sol-gel method is currently the main method for preparing it. The process generally involves stirring ethyl orthosilicate and anhydrous ethanol in a certain molar ratio to form a uniform mixed solution, slowly adding an appropriate amount of deionized water while stirring, then adjusting the pH value of the solution, and then adding a suitable surfactant. The resulting solution is stirred and aged at room temperature to obtain a gel, and then the required SiO2 powder is obtained through steps such as drying.
The silica microspheres prepared by the sol gel method have good dispersibility and controllable size, and because the silicon hydroxyl groups on the surface of silica are very suitable to be used as the bridge for modification, making them functional, the constantly developing modification technology provides new opportunities for their increasingly expanded application fields, such as using monodisperse silica microspheres as the core or shell to prepare some materials with excellent performance.
- Template method
The template method is an important method for preparing this microspheres. It mainly uses surfactants as templates, and alternately adsorbs polyelectrolytes of opposite charges and SiO2 particles of different particle sizes on them to generate nano-silica microspheres. The resulting product is then calcined at high temperature to obtain nano-silica microspheres with a porous structure.
The traditional template method for preparing nano-silica microspheres is complex, the shell structure of the resulting hollow silica microspheres is relatively loose and easy to break, and the conditions are relatively harsh during the process, and the morphology of the resulting hollow microspheres is difficult to control. The shell thickness and morphology of the composite microspheres generated by the improved template method are easy to control, and the surface of the resulting composite microspheres is uniform and the structure is dense.
- Precipitation method
The precipitation method is to mix the reactant solution with other auxiliary agents, and then add an acidifier to the mixed solution for precipitation. The resulting precipitate is then dried and calcined to obtain nano-silica microspheres. The process of preparing silica microspheres by chemical precipitation method is simple, with a wide range of raw material sources, low requirements for experimental equipment, low energy consumption, and simple process. However, its product properties are difficult to control and are affected by many variable factors, which requires further in-depth research.
- Supergravity method
The supergravity reaction method for preparing this microspheres is to use the transfer and micro-mixing process between phases in the supergravity field to be strengthened as much as possible, thereby shortening the reaction time and greatly improving the reaction rate. The process of preparing nano-silica microspheres by supergravity is simple, the raw materials are easy to obtain, and in the supergravity environment, the mass transfer process and micro-mixing process are greatly strengthened, greatly shortening the reaction time. However, this method has high requirements for the reactor and is costly.
- Microemulsion method
Reverse microemulsion method is an important method for preparing this developed in recent years. In W/O micro lotion, it is generally composed of surfactant, cosurfactant, oil (usually organic matter with small polarity) and water. In the system, surfactants surround the water phase and disperse in a continuous oil phase, and the surrounded water core is an independent “microreactor”. Due to the controlled reaction in the water core, the prepared nanoparticles have advantages such as good particle dispersion, narrow particle size distribution, and easy regulation compared to traditional methods.
The reaction of preparing nano silica particles by micro lotion method is controlled in the water core, and the size and shape of the product particles are closely related to the size of the water core. Compared with traditional preparation methods, microemulsion method is more convenient for controlling the particle size of nano silica microspheres, and the resulting particles have good dispersibility. Therefore, microemulsion method has broad prospects in the preparation of ultrafine nano silica microspheres.
- Gas phase method
The gas phase method for preparing this microspheres is to hydrolyze halosilanes (such as silicon tetrachloride, silicon tetrafluoride, methyl silicon trichloride, etc.) at high temperature in a hydrogen-oxygen flame to generate silica particles.
7.Other methods
Due to the increasing demand for this microspheres in recent years, their application scenarios and preparation methods have also varied. Decher et al. prepared nano silica microspheres using layer by layer self-assembly (LBL) method, with the core being layer by layer deposition through adsorption physical properties. There are many methods for preparing it in a hypergravity experimental environment. The reaction efficiency is greatly improved, hence it is called the supergravity reaction method. The process is simple, but it requires high experimental equipment and has a relatively large particle size, which limits its development.
At present, the preparation of this microspheres has developed into a complete system, but various methods have their own advantages and disadvantages. The low yield and high cost of sol gel method limit its application and is only suitable for laboratory preparation. In recent years, methods such as gas-phase and plasma oxidation have also received a certain degree of attention, all of which are dedicated to solving the problem of low yield. However, there is still a certain gap in the controllability, dispersibility, and controllability of microspheres compared to traditional methods.
The particle size selection range of silica microspheres is wide, ranging from 0.1um to 1um. This type of microsphere has low non-specific adsorption properties for biomolecules and does not adsorb proteins, making it particularly suitable for biomedical applications such as immunoassays. In addition, the particle size of it is highly uniform, with small inter batch differences and good repeatability.
Silica microspheres can be used in scientific research fields, such as the preparation of streptavidin labeled silica microspheres/silk fibroin loaded mesoporous silica hollow microspheres. In the industrial field, it also have a wide range of applications, such as catalyst carriers, adsorbents, fillers, cosmetics, and coatings.
Silica microspheres naturally have high hydrophilicity, so the non-specific adsorption of proteins on them should be low. The higher density of silica (2.0 g/mL for PS and 1.05 g/mL for PS) results in a significant difference in settling velocity. Due to the fact that sedimentation in water depends on the difference between microsphere density and water density (silica is 2.00-1.00=1.00, while PS is 1.05-1.00=0.05), the sedimentation rate of silica microspheres is approximately 0 times that of PS!
This important difference may lead to some interesting tests and determinations, as aggregated microspheres settle faster. Small microspheres can be used for agglutination tests. Unaggregated microspheres will remain suspended, but aggregated microspheres will quickly fall out of the solution.