Introduction to Magnetic Microspheres
At present, the application of magnetic microspheres in biomedicine is represented by immunomagnetic microspheres, which have developed rapidly and have already been launched with many successful applications. Magnetic nanomaterials are an important component of magnetic microspheres, therefore the application of magnetic nanoparticles should also be considered.
- Preparation and Properties of Magnetic Nanomaterials
The commonly used magnetic materials are ferric oxide, ferric oxide, iron cobalt alloy, etc. These magnetic materials have good magnetic responsiveness and can be easily obtained at the nanoscale using appropriate methods. For example, dissolve a certain amount of magnetic material in an appropriate amount of distilled water, filter and mix, dilute with a certain amount of distilled water, stir evenly, and add an appropriate surfactant as a dispersant. At a certain temperature, keep stirring and add the solution to the system at a certain speed.
After dropwise addition, continue stirring for half an hour. Take it out and place it on a magnet to allow the iron oxide particles to settle, remove the upper clear liquid, then add an appropriate amount of dispersant solution in water, disperse with ultrasound, filter, and obtain the color colloid solution of iron oxide magnetic nanoparticles.
The concentration of alkaline solution during the preparation process has a significant impact on the properties of iron oxide nanoparticles. A low concentration of alkaline solution results in a lower magnetization strength of the magnetic material; The dripping speed of alkaline solution affects the performance of magnetic materials. The slower the dripping speed, the smaller the particle size of the magnetic material and the lower the magnetization strength; The reaction temperature of the system also has an impact, as temperature increases particle size and enhances magnetization.
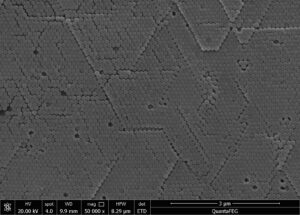
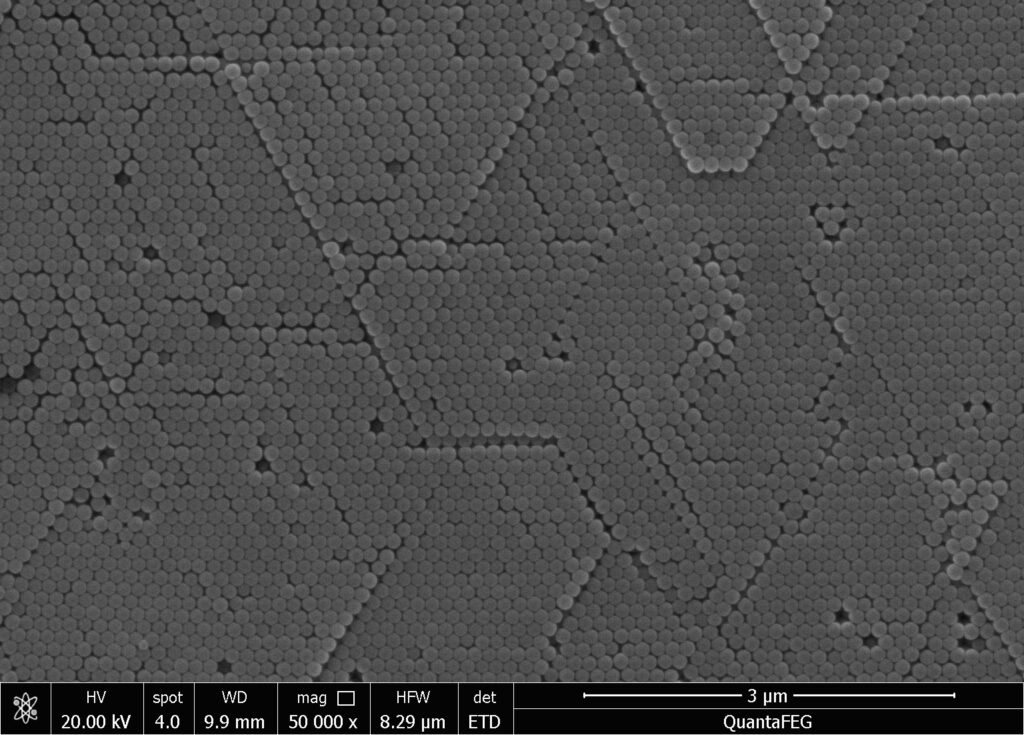
The magnetic nanomaterials obtained by the above method can be diluted with water appropriately and photographed under an electron microscope. The particle size and distribution can be determined by an image analyzer, or directly measured by a laser particle size analyzer. Different sizes of nanomaterials can be obtained, with the minimum average particle size being a few nanometers. The particle size is generally normally distributed. The structure of magnetic nanomaterials can also be analyzed using an X-ray diffraction analyzer, and their magnetization can be measured using a magnetometer.
- Preparation of magnetic microspheresusing magnetic nanomaterials
Magnetic microspheres can be prepared from magnetic nanoparticles and polymer skeleton materials. The polymer materials include polystyrene, silane, polyene, polyacrylic acid, starch, pectin, gelatin, albumin, ethyl cellulose, etc. There are both natural and synthetic materials, which can be used alone or in combination as skeleton materials. These skeleton materials should have stable properties, high strength, and no toxic side effects.
The methods for preparing magnetic microspheres can be divided into one-step and two-step methods: the one-step method involves adding magnetic nanomaterials before ball formation, and during ball formation, the polymer wraps them inside; The two-step method involves first preparing non-magnetic microspheres, then introducing magnetic materials into them through processing, and finally dispersing magnetic nanoparticles into the bone reinforcement material of the microspheres.
The one-step method developed earlier and there are many methods, but only the following four are introduced.
(1)Disperse magnetic nanomaterials (such as iron oxide nanoparticles) in water, add monomers of polymer, and then add initiators to initiate polymerization reaction under appropriate conditions, so that the monomers polymerize around the iron oxide nanoparticles to form magnetic microspheres. Magnetic microspheres with synthetic polymer materials as the skeleton are often prepared using this method.
(2)Disperse magnetic nanomaterials in an aqueous solution of polymer skeleton materials, add appropriate surfactants, and emulsify them into W/O emulsions in a hydrophobic solvent. Use thermal curing or cross-linking curing methods to solidify the polymer skeleton materials into magnetic microspheres. Magnetic microspheres with natural polymer materials as the skeleton are often prepared using this method.
(3)Firstly, Fe2+and Fe3+are precipitated in alkaline solution to form superparamagnetic iron oxide, and then coated with silane to form microspheres. The prepared silane magnetic microspheres can be dispersed in aqueous media without rapid precipitation, and can be easily recovered using a magnetic field.
- By incorporating magnetite itself as a part of the oxidation-reduction system, polymers can completely envelop magnetite. Polymers are initiated by iron ions that diffuse from magnetite particles and become free radicals through the reduction of persulfate. A water-borne gel magnetic microspherescontaining acrylic resin can be prepared by this method.
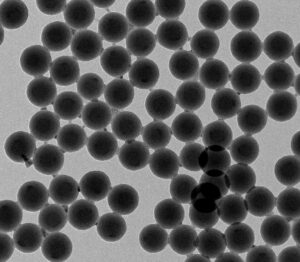
Amino magnetic beads
- Preparation, properties, and principles of action of immunomagnetic microspheres
(1)Preparation of Immunomagnetic Microspheres
Immunomagnetic microspheres (IMMS), also known as immunomagnetic beads (IMB), are magnetic microspheres with monoclonal antibodies bound to their surfaces. Due to the need to bind appropriate antibodies to the surface of magnetic microspheres, it is required that the magnetic microspheres used can bind to monoclonal antibodies through their surface chemical genes or have a strong surface adsorption force to firmly bind to monoclonal antibodies. Cross linked polystyrene microspheres have high strength and are easily chemically modified on the surface, making them an ideal skeleton material for preparing immunomagnetic microspheres.
There are two forms of connection between microspheres and antibodies: adsorption binding and covalent binding. Adsorption binding relies on the non-specific adsorption force of the microsphere surface on the antibody, while covalent binding relies on the covalent reaction between the active groups on the microsphere surface and the antibody. Adsorption binding can only be relatively strong when the microspheres have a very large surface area. Therefore, for microspheres with a relatively flat surface, surface treatment is necessary to improve their antibody binding strength to ensure that the IMMS surface has sufficient antibodies.
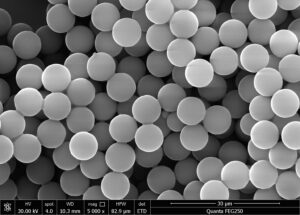
After surface treatment, magnetic microspheres are cultured with monoclonal antibodies in appropriate buffer solution. The antibodies quickly bind to the magnetic microspheres through physical adsorption. If there are active groups on the surface of the microspheres, they will then covalently bind to the surface of the magnetic microspheres through slower chemical reactions.
(2)Performance and principle of action of immunomagnetic microspheres
Immunomagnetic microspheres are mainly used for cell separation and other applications. Due to its ability to specifically bind to the target substance and make it magnetic responsive, the free magnetic microspheres were co cultured with a complex mixture containing the target substance (the substance to be separated). Immune microspheres can selectively bind to target substances through antigen antibody reactions. When this compound passes through a magnetic field device, the target substance bound to the immune magnetic microspheres will be retained by the magnetic field and separated from other complex substances.
Immune magnetic microspheres used for cell separation have the following conditions: stable chemical properties and no aggregation; Not non-specific binding with cells; The binding between magnetic microspheres and antibodies is firm; The size of magnetic microspheres is uniform, the magnetic responsiveness is good, and the content of magnetic nanomaterials is uniform and consistent; Magnetic microspheres are of appropriate size and are not easily engulfed by cells.
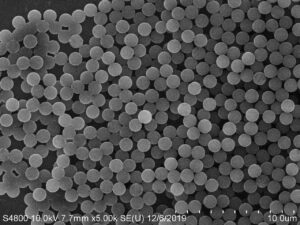
The binding of IMM to cells can be achieved through two methods: direct and indirect. The direct method refers to the antibody being sequentially attached to magnetic microspheres and then binding to target cells. Indirect method refers to first mixing cells with specific antibodies for cultivation, allowing the specific antibodies to bind to the cell surface, and then adding magnetic microspheres pre treated with anti mouse IgG (secondary antibody). Indirectly bind magnetic microspheres to target cells. The direct method can reduce washing and cultivation steps, but it is rarely used for IgM monoclonal antibodies. Compared with direct method, indirect method,
In addition to a suitable range, a set of monoclonal antibodies can also be used to achieve better cell clearance effects. But after multiple washing steps, the specificity will also decrease.
The development of monoclonal antibodies targeting monocytes has made it possible to isolate cells with specific surface markers. There are generally three different methods, namely flow cytometry technology (PACS), allogeneic red blood cell garland technology with surface attached secondary antibodies, and Panning technology that passively adsorbs antibodies on polystyrene tissue plates. These technologies all have their own drawbacks.
FACS is expensive, technically complex, and often plagued by issues such as the capacity, activity, and sterility of the sorted cells; The red blood cell garland technique cannot handle a large number of cells, and there is currently no mature and convenient method to couple antibodies to the red blood cell membrane; Panning technology also has many limitations, such as difficulty in scaling up, cumbersome steps, inability to quantify, and target cells often mixed with non-specific antibodies adsorbed on other cells at the bottom of the culture plate.
Relatively speaking, immunomagnetic microspheres have the advantages of easy operation, rapid and complete separation, and high cell purity, especially in terms of ease of operation and time saving, which are difficult to compare with other separation methods.