Time-resolved fluorescence beads, as a special type of functional microspheres, are polystyrene microspheres internally embedded with complexes of rare earth ions. The rare earth ions commonly used for internal embedding include Eu3+, Tb3+, Sm3+, and Dy3+. When these rare earth ions form complexes with certain ligands such as β-diketones and aromatic amines, they can absorb ultraviolet light and emit strong characteristic fluorescence from the rare earth ions.
1. Internal Embedding Process
Time-resolved fluorescence beads employ an internal embedding process for fluorescent dyes. This technique effectively prevents the leakage of fluorescent dyes and ensures optimal surface activity when coupled with biomolecules. Each bead is internally embedded with numerous fluorescent molecules, thereby significantly enhancing the fluorescence intensity and sensitivity of the analysis.
2. Rare Earth Ion Ligand
Due to the low luminescence efficiency of rare earth ions themselves, when rare earth ions form complexes with ligands that have high absorption coefficients, the ligands absorb the laser energy and transfer the energy to the rare earth ions. Upon receiving the transferred energy, the rare earth ions are excited to a resonant energy level and emit fluorescence during the transition from the resonant energy level back to the ground state. This emission exhibits strong characteristic fluorescence of the rare earth ions.
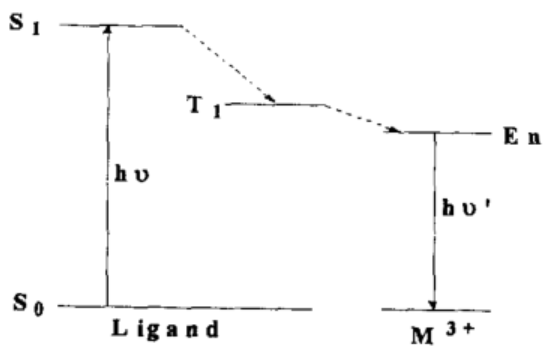
Luminescence mechanism of rare earth ion complexes
(S0 represents the ground state of the ligand, S1 represents the lowest excited singlet state of the ligand, T1 represents the ligand’s excited state, and En represents the excited state of the rare earth ion.)
Based on the microsphere structure and luminescence mechanism described above, time-resolved fluorescence beads have the following characteristics compared to conventional fluorescence beads:
Internal embedding process:
No dye on the surface, facilitating coupling.
Fluorescence intensity:
Higher fluorescence intensity compared to regular red or green fluorescence microspheres.
Stokes shift:
Larger Stokes shift compared to regular fluorescence microspheres (mostly above 250nm).
Half-life:
Long fluorescence lifetime, providing strong resistance to interference.
(Time-resolved fluorescence lifetimes are typically above 100μs, which is several orders of magnitude higher than that of regular fluorescence microspheres. This is due to the energy transfer through the excited state of the ligands in rare earth complexes.)
Stability:
The stability of rare earth ion complexes is higher compared to regular fluorescence, resulting in more consistent batch-to-batch performance.
Currently, the most common time-resolved fluorescence beads are internally embedded with the rare earth element Europium (Eu). These beads are widely used in the development of fluorescence quantitative immunoassay test strips for Point-of-Care Testing (POCT) applications. They find extensive use in personal health management, clinical diagnostics, infectious disease, and epidemic detection, on-site law enforcement testing (such as alcohol testing, drug testing, and forensic toxicology), and food safety testing. Analytes can be detected rapidly and quantitatively with quantitative fluorescence detection instruments.

