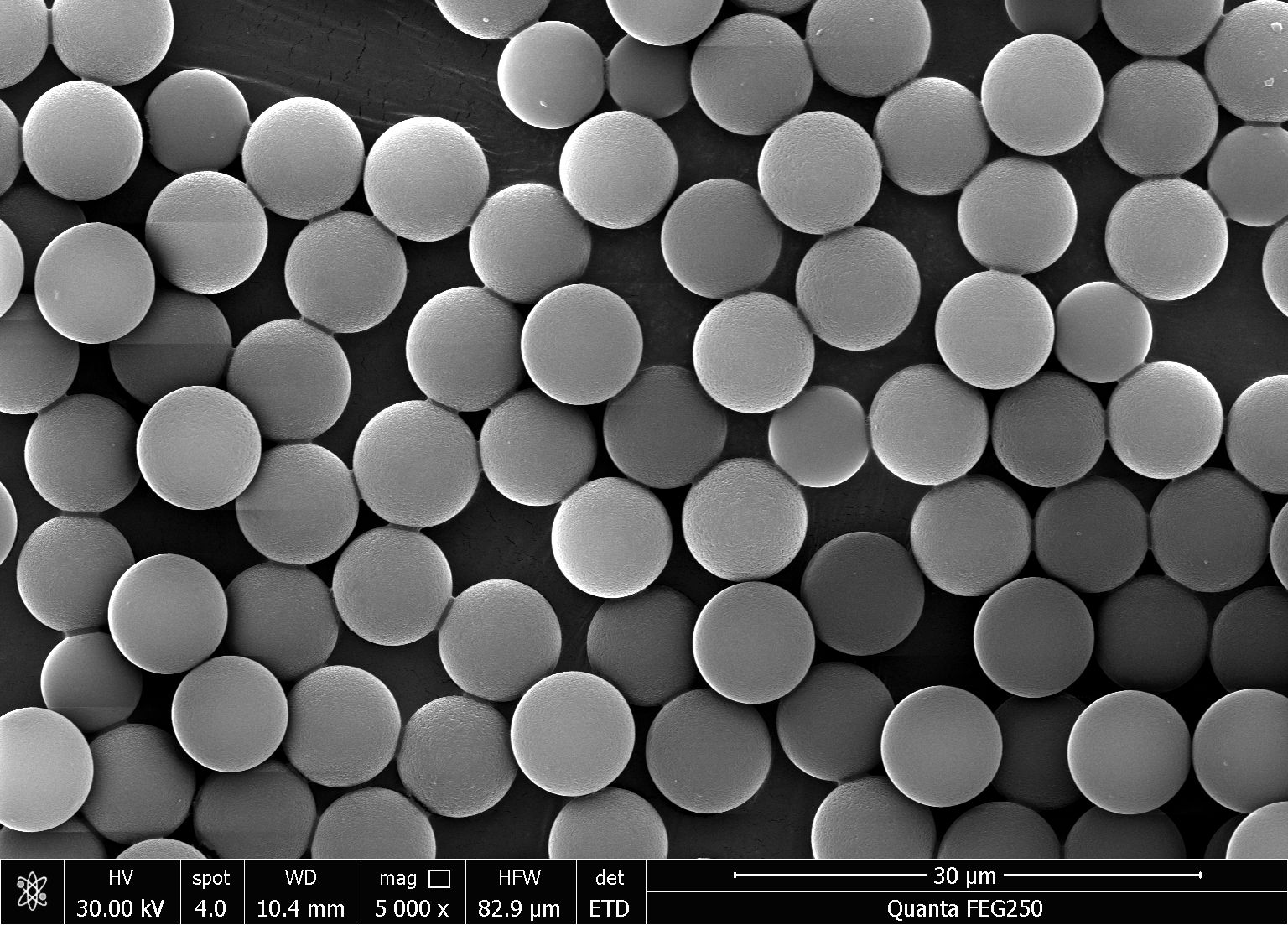
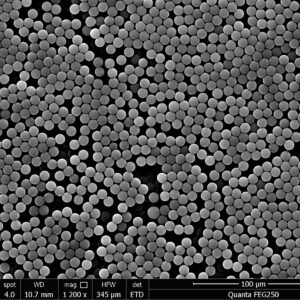
Product Category
Hot Products
-
 Fluorescent Carboxyl Microspheres
评分 0 / 5
Fluorescent Carboxyl Microspheres
评分 0 / 5 -
 PMMA Microspheres
评分 0 / 5
PMMA Microspheres
评分 0 / 5 -
 Silica Microspheres
评分 0 / 5
Silica Microspheres
评分 0 / 5 -
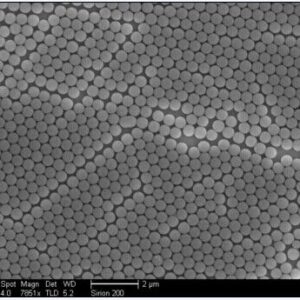 Immune Turbidimetric Microspheres
评分 0 / 5
Immune Turbidimetric Microspheres
评分 0 / 5
Nickel Magnetic Beads
SHBC nickel magnetic beads combine traditional magnetic microspheres and nickel chromatography media. Nickel magnetic beads are a new generation of functional magnetic beads designed and developed for the rapid separation of proteins with histidine tags. The magnetic beads are based on agarose, which is electrically neutral, hydrophilic and has good biocompatibility, avoiding non-specific adsorption of proteins due to charge or hydrophobicity.
1.Nickel Magnetic Beads Overview
SHBC nickel magnetic beads combine traditional magnetic microspheres and nickel chromatography media. Nickel magnetic beads are a new generation of functional magnetic beads designed and developed for the rapid separation of proteins with histidine tags. The magnetic beads are based on agarose, which is electrically neutral, hydrophilic and has good biocompatibility, avoiding non-specific adsorption of proteins due to charge or hydrophobicity.
Compared with traditional nickel chromatography media, nickel magnetic beads are used to separate and purify proteins with histidine tags. There is no need to perform any operations on the crude protein (inclusion bodies need to be denatured), and no column chromatography equipment is required. It is easy to operate and has better cost performance.
2.Product features
Low non-specific adsorption
Good purification effect for small volume samples
Easy to conduct screening experiments and highly reproducible
Easy to scale up or down the scale of protein purification
High protein loading capacity and high protein purity after purification
3.Scope of application
Isolate or purify proteins with histidine tags
4.product parameters
| Particle size | 1um, 3um, 5um, 10um, 20um, 30 μm, 40um, 50um |
| Matrix | Matrix polymer magnetic beads |
| Ligand | IDA-Ni |
| Loading capacity | ≥40 mg His-tagged Protein/mL (magnetic bead sedimentation volume) |
| Use temperature | Use temperature room temperature |
| Storage conditions | 2-8°C, stored in 20% ethanol |
| Shelf life | 5 years |
5.How to use (can be adjusted according to actual situation)
Magnetic beads bind to proteins
Put an appropriate amount of magnetic beads into a centrifuge tube, wash them three times with Binding Buffer, and redisperse them with Binding Buffer. Mix the broken and lysed bacterial liquid with the magnetic beads, and mix on a mixer for 30 minutes.
Wash magnetic beads
After 30 minutes, place the centrifuge tube on a magnetic separator to separate the magnetic beads and remove the supernatant for detection. Add the Washing Buffer to the magnetic beads, turn over several times to resuspend the magnetic beads, separate magnetically, and take out the Washing Buffer for detection.
Add Washing Buffer to resuspend the magnetic beads, and transfer the magnetic beads to a new centrifuge tube (to avoid protein contamination). Magnetic separation, take out and merge the Washing Buffer.
Target protein elution
Users can change the elution volume to adjust the concentration of the target protein as needed. Add Elution buffer, gently flip the centrifuge tube to suspend the magnetic beads, and collect the eluate into a new centrifuge tube, which is the purified target protein. Repeat several times to ensure complete elution of the target protein.
Regeneration of magnetic beads
After using magnetic beads three times, the capacity may be significantly reduced, and it is recommended to regenerate them. If you need to regenerate magnetic beads, you can do the following:
a) Disperse the magnetic beads in phosphate buffer (20 mM, pH 7.4) containing 0.1 M EDTA, shake at room temperature for 10 minutes, discard the supernatant by magnetic suction, and repeat once.
b) Wash the magnetic beads with high-purity water several times to ensure that there is no EDTA in the solution.
c) Wash the magnetic beads with 0.5 M NaOH and 2 M NaCl solution, shake at room temperature for 5 minutes, magnetically absorb, discard the supernatant, and wash the magnetic beads with high-purity water until the washing solution becomes neutral.
d) Use 0.1 M NiSO4 solution to disperse the magnetic beads, and shake at room temperature for 10 minutes. Discard the supernatant by magnetic suction, and wash the magnetic beads several times with high-purity water until there are no Ni ions in the solution. Store magnetic beads in 20% ethanol.
6.Things to note
Magnetic beads have a high density and will easily sink if left standing for a long time. Therefore, please shake well before use to obtain a uniform magnetic bead suspension.
Appendix (Solvent Tolerance Table)
| Solvent name | Tolerable concentration | Solvent name | Tolerable concentration | Solvent name | Tolerable concentration |
| DTE | 5 mM | Triton X | 2% | Tris-HCl pH7.4 | 100 mM |
| DTT | 5 mM | Tween 20 | 2 % | Imidazole | 1 M |
| TCEP | 5 mM | NP-40 | 2 % | Ethanol | 20 % |
| Urea | 8 M | CHAPS | 1 % | EDTA | 1 mM |
| Guanidine•HCl | 6 M | PB pH 7.4 | 50 mM | Citrate | 60 mM |
| Product Number | Product Name | Particle Size |
| MagNi1UM-10 | Nickel Magnetic Beads | 1μm |
| MagNi3UM-10 | Nickel Magnetic Beads | 3μm |
| MagNi5UM-10 | Nickel Magnetic Beads | 5μm |
| MagNi10UM-10 | Nickel Magnetic Beads | 10μm |
| MagNi20UM-10 | Nickel Magnetic Beads | 20μm |
| MagNi30UM-10 | Nickel Magnetic Beads | 30μm |
| MagNi40UM-10 | Nickel Magnetic Beads | 40μm |
| MagNi50UM-10 | Nickel Magnetic Beads | 50μm |
相关产品
-
Hot Sale products
PMMA Microspheres
-
Hot Sale products
Immune Turbidimetric Microspheres
-
Fluorescent microspheres
Amino Red Fluo Microspheres
-
Hot Sale products
Tosyl Magnetic Beads