Product Category
Hot Products
-
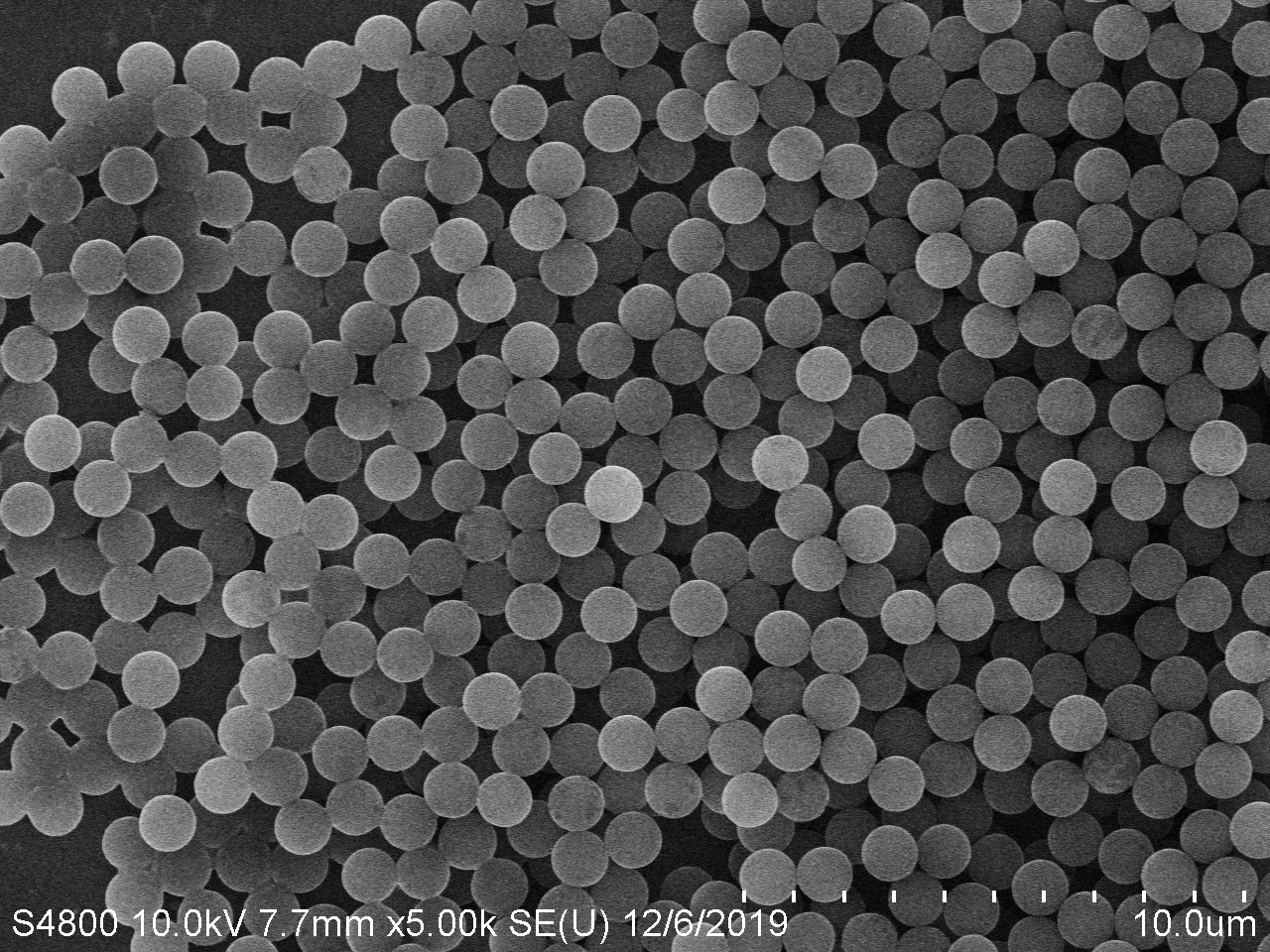
 Fluorescent Carboxyl Microspheres
评分 0 / 5
Fluorescent Carboxyl Microspheres
评分 0 / 5 -
 PMMA Microspheres
评分 0 / 5
PMMA Microspheres
评分 0 / 5 -
 Silica Microspheres
评分 0 / 5
Silica Microspheres
评分 0 / 5 -
 Immune Turbidimetric Microspheres
评分 0 / 5
Immune Turbidimetric Microspheres
评分 0 / 5
Carboxy Magnetic Beads
MagCOOH carboxyl magnetic beads use superparamagnetic Fe3O4 as the core and are modified with carboxyl functional groups on the surface. They have the characteristics of high magnetic content and monodispersity. Under the action of specific reagents, the abundant carboxyl functional groups can covalently bind biological ligands such as proteins to the surface of magnetic beads, thereby achieving rapid separation and enrichment of target substances from samples. This is an important issue in molecular biology, An important carrier for research in medicine and other related fields.
1.Carboxy Magnetic Beads Overview
MagCOOH carboxy magnetic beads use superparamagnetic Fe3O4 as the core and are modified with carboxyl functional groups on the surface. They have the characteristics of high magnetic content and monodispersity. Under the action of specific reagents, the abundant carboxyl functional groups can covalently bind biological ligands such as proteins to the surface of magnetic beads, thereby achieving rapid separation and enrichment of target substances from samples. This is an important issue in molecular biology, An important carrier for research in medicine and other related fields.
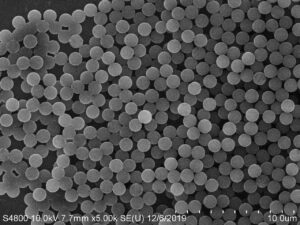
2.Product features
Fast magnetic response and highly uniform particle size
Small batch-to-batch variation and high repeatability
Good biocompatibility and low cytotoxicity
Convenient to couple with a variety of biomolecules and easy to operate
3.Scope of application
Targeted drugs
Biologically active substance detection
Sample enrichment and separation
4.Product parameters and specifications
Particle size: 200nm, 1um, 3um, 5um, 10um, 20um, 30um, 50um
Particle size uniformity: CV<5%
Concentration: 50 mg/mL
Solvent: DI Water
Surface functional group: carboxyl group (-COOH)
Carboxyl density: 200 μmol/g
Applicable solutions: PBS, Tris-HCl, H2O
Iron (Fe) content: 30%
5. Coupling method with biomolecules (EDC/NHS method)
Activation of carboxyl groups on the surface of magnetic beads
After mixing the magnetic beads, remove an appropriate amount of magnetic beads into a centrifuge tube, magnetically remove the supernatant, and wash the magnetic beads with the prepared 20 mM MEST buffer (20 mM MES buffer, pH 5.0, 0.05% Tween 20) 3 Second-rate. Take an appropriate amount of newly prepared EDC solution and an equal volume of NHS solution to disperse the magnetic beads, and activate them at 25°C for 3 hours.
Magnetic beads coupled with biomolecules
Magnetically absorb the magnetic beads, discard the supernatant, disperse an appropriate amount of biomolecules in a pH 8.0 buffer, redisperse the magnetic beads in this solution, and couple at 25°C for 3 hours or 4°C overnight. After coupling, magnetically absorb the beads, remove the supernatant, add buffer containing 1% BSA, redisperse the beads, and couple at 25°C for 1 hour to block unreacted activated carboxyl groups.
After blocking, magnetically absorb the beads, remove the supernatant, wash the beads 3 times with 20 mM pH 7.4 PBS buffer, redisperse, and store at 4°C. If necessary, add 0.02% sodium azide to inhibit bacterial growth.
6.things to note
Magnetic beads have a high density and will easily sink if left standing for a long time. Therefore, please shake well before use to obtain a uniform magnetic bead suspension.
7.Storage conditions and expiry date
Storage conditions: Store at 2~8℃, do not freeze or dry.
| Product Number | Product Name | Particle Size |
| MagCOOH100-10 | Carboxy Magnetic Beads | 100nm |
| MagCOOH200-10 | Carboxy Magnetic Beads | 200nm |
| MagCOOH500-10 | Carboxy Magnetic Beads | 500nm |
| MagCOOH1UM-10 | Carboxy Magnetic Beads | 1μm |
| MagCOOH2UM-10 | Carboxy Magnetic Beads | 2μm |
| MagCOOH3UM-10 | Carboxy Magnetic Beads | 3μm |
| MagCOOH5UM-10 | Carboxy Magnetic Beads | 5μm |
| MagCOOH10UM-10 | Carboxy Magnetic Beads | 10μm |
| MagCOOH20UM-10 | Carboxy Magnetic Beads | 20μm |
| MagCOOH30UM-10 | Carboxy Magnetic Beads | 30μm |
| MagCOOH50UM-10 | Carboxy Magnetic Beads | 50μm |
| MagCOOH100UM-10 | Carboxy Magnetic Beads | 100μm |
相关产品
-
Fluorescent microspheres
Fluorescent Carboxyl Microspheres
-
Hot Sale products
Silica Microspheres
-
Hot Sale products
Streptavidin Microspheres
-
Hot Sale products
Tosyl Magnetic Beads