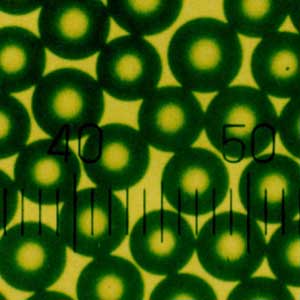
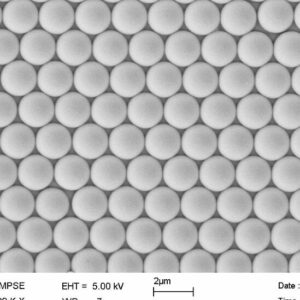
Product Category
Hot Products
-
 Fluorescent Carboxyl Microspheres
评分 0 / 5
Fluorescent Carboxyl Microspheres
评分 0 / 5 -
 PMMA Microspheres
评分 0 / 5
PMMA Microspheres
评分 0 / 5 -
 Silica Microspheres
评分 0 / 5
Silica Microspheres
评分 0 / 5 -
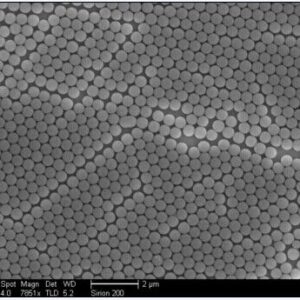 Immune Turbidimetric Microspheres
评分 0 / 5
Immune Turbidimetric Microspheres
评分 0 / 5
Time-resolved Fluorescent Microspheres
Time resolved fluorescence microspheres is designed for lateral flow immunoassay reagents and is made of time-resolved fluorescent dyes copolymerized with styrene. It has the characteristics of large stokes displacement, no internal quenching effect during aggregation, strong detection signal, fluorescent dye wrapped in microspheres, not easily affected by the external environment, and stable fluorescence. It is an ideal marker for immunofluorescence quantitative chromatography. 2. Microsphere parameters
1. Time Resolved Fluorescent Microspheres Product introduction
Time resolved fluorescent microspheres is designed for lateral flow immunoassay reagents and is made of time-resolved fluorescent dyes copolymerized with styrene. Time-resolved fluorescence microspheres has the characteristics of large stokes displacement, no internal quenching effect during aggregation, strong detection signal, fluorescent dye wrapped in microspheres, not easily affected by the external environment, and stable fluorescence. It is an ideal marker for immunofluorescence quantitative chromatography.
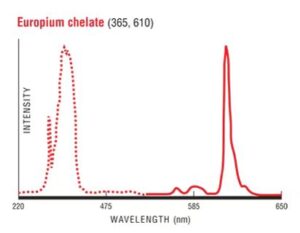
2. Microsphere parameters
1. Ingredients: Modified polystyrene microspheres modified with fluorescent complexes of rare earth element europium
2. Solid content: 1% (1ml suspension contains 10mg microspheres)
3. Average particle size: 200nm
4. Particle size uniformity: CV<5%
5. Functional groups: carboxyl group, sulfate group, etc.
6. Carboxyl content: 200±10μmol/g
7. Fluorescence properties: excitation wavelength: 365nm, emission wavelength: 610nm
8. Fluorescence lifetime: 720μs
9. Density: 1.05g/cm3
10. Refractive index: 1.59 (589nm, 25℃)
11. Appearance: white emulsion
Three: Microsphere preservation
Storage conditions: Store at 2-8°C, protected from light, sealed, and cannot be frozen and thawed repeatedly. 4. How to use
(1) Activation steps:
1. Disperse the microspheres ultrasonically for about 2 minutes, and then shake them manually;
2. In a 2mL EP tube, add 100μL microspheres (solid content 1%), then add 900μL ultrapure water (or MES buffer) and set aside;
3. Preparation:
10mg/ml N-hydroxysuccinimide (NHS) ethanol solution A solution
10mg/ml 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) ethanol solution B
Note: NHS and EDC are both easy to absorb moisture. These two reagents need to be rewarmed for more than 30 minutes before weighing.
4. Add 5 μl of solution A to the microsphere suspension and mix well, then add 5 μl of solution B, mix again, and react at room temperature for 15 to 30 minutes;
5. Centrifuge at 14000r/min for 15min, remove the supernatant, add 1ml of ultrapure water (or antibody coupling buffer), and then disperse evenly by ultrasonic.
Note: Be sure to disperse thoroughly, otherwise the microspheres after cross-linking the antibody will easily agglomerate and the strip will run poorly.
(2) Antibody cross-linking
Pay special attention to whether the antibody used for cross-linking precipitates. If precipitation occurs, the antibody needs to be centrifuged or filtered, otherwise the cross-linked microspheres will agglomerate. Antibodies that have been filtered or centrifuged need to be re-determined for antibody concentration.
The amount of cross-linked antibody on the surface of microspheres is: antibody/microsphere = 10~50μg/mg, and the optimal labeling amount is generally 20μg/mg.
Antibody cross-linking steps:
1. Take 1ml of the above activated microsphere suspension, disperse it by ultrasonic, and add the antibody dropwise while stirring. The optimal antibody dosage is 20ug;
Note: The recommended antibody cross-linking buffer is boric acid buffer, 0.02mol/L, pH=8.0. Each antibody has its optimal cross-linking buffer (type, concentration, pH), which you need to explore by yourself.
2. Mix at room temperature for 1 hour, and then test the performance of the microspheres after coupling the antibody;
Note: Goat anti-mouse IgG-coated test strips can be used to test the fluidity and antibody recognition performance of the microspheres after antibody cross-linking.
3. Add BSA (final concentration at 0.5%) for blocking for 1 hour;
4. Centrifuge at 13000r/min for 15min;
5. Add 1ml of preservation solution to the centrifuged microspheres, mix well, and store in a refrigerator away from light until use.
Note: Because the antibodies of each project are different, you need to explore the best preservation solution yourself. The basic preservation solution formula is: 10% sucrose (w/v), 0.01mol/L PB pHPB pH=7.4.
| Product Number | Product Name | Particle Size |
| EU070-10 | Time resolved fluorescent Microspheres | 70nm |
| EU100-10 | Time resolved fluorescent Microspheres | 100nm |
| EU200-10 | Time resolved fluorescent Microspheres | 200nm |
| EU300-10 | Time resolved fluorescent Microspheres | 300nm |
| EU400-10 | Time resolved fluorescent Microspheres | 400nm |
相关产品
-
Hot Sale products
PMMA Microspheres
-
Fluorescent microspheres
Colored Carboxyl Microspheres
-
Fluorescent microspheres
Fluo Red-Fluo Microspheres
-
Fluorescent microspheres
Quantum Dot Polystyrene Microspheres